Resources
Learn more about what Greenbrook can offer you and your patient
treat depression at the source
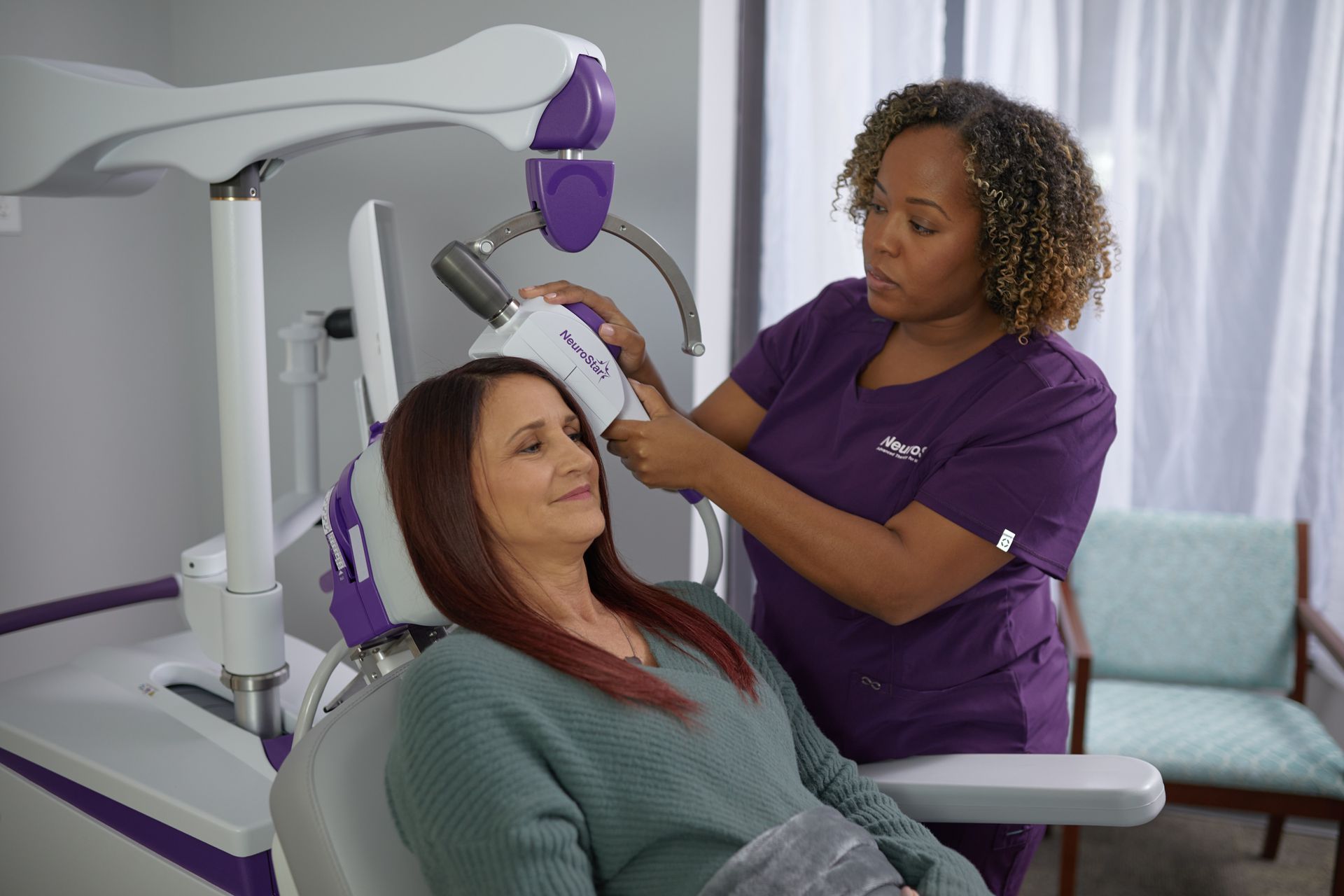
NeuroStar TMS Therapy
NeuroStar is an FDA-cleared non-drug, non-invasive treatment for patients with depression who aren’t satisfied with the results of antidepressant medications. NeuroStar is proven safe, proven effective, and proven to transform lives.4
Mechanism of Action
Unlike medications that travel through the entire body through the bloodstream, NeuroStar focuses treatment directly at the source—the brain. It uses focused magnetic pulses to reignite dormant synapses in the brain.5,6
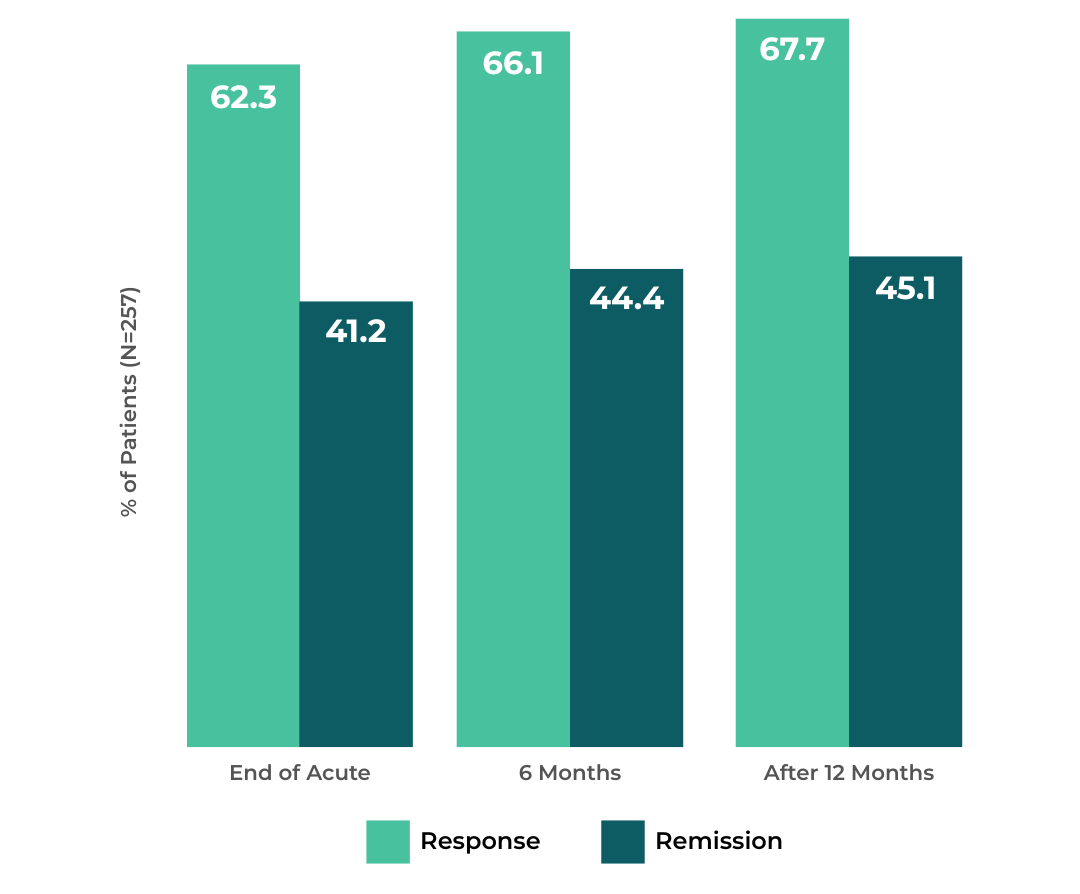
Proven Depression Relief That Lasts
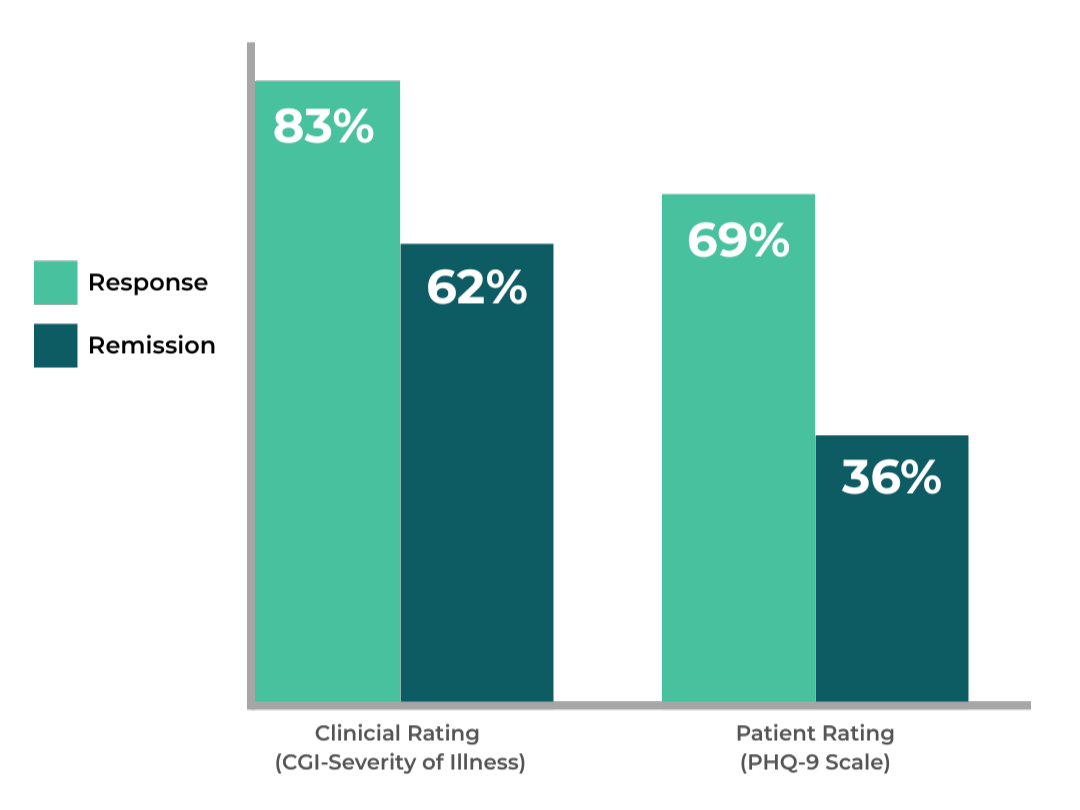
Who is NeuroStar TMS For?
For patients who haven’t seen adequate relief from antidepressants, trying another medication isn’t always the answer. NeuroStar may be the best option: a non-drug depression treatment proven to transform lives.
Identifying a potential NeuroStar TMS Candidate
- Has a diagnosis of adult MDD or OCD
- Has a diagnosis of adolescent MDD (ages 15-21)
- Unhappy with current medication therapy
- Has one or more medication failures
- Cannot tolerate medication side effects
- Wishes to avoid antidepressants
treat depression with a
different approach
SPRAVATO®
SPRAVATO®
(esketamine) nasal spray is FDA-approved for treatment resistant depression, esketamine nasal spray treatment is a novel approach that provides rapid symptom relief by targeting NMDA receptors, often effective within weeks.2,3
Mechanism of Action
SPRAVATO® is different because it acts on the glutamate pathway. The primary antidepressant activity of SPRAVATO® is not believed to directly involve inhibition of serotonin or norepinephrine reuptake. The precise mechanism of action (MOA) is unknown.9-12 SPRAVATO® may increase Brain Derived Neurotrophic Factor (“BDNF”), which enhances neuroplasticity.13-16
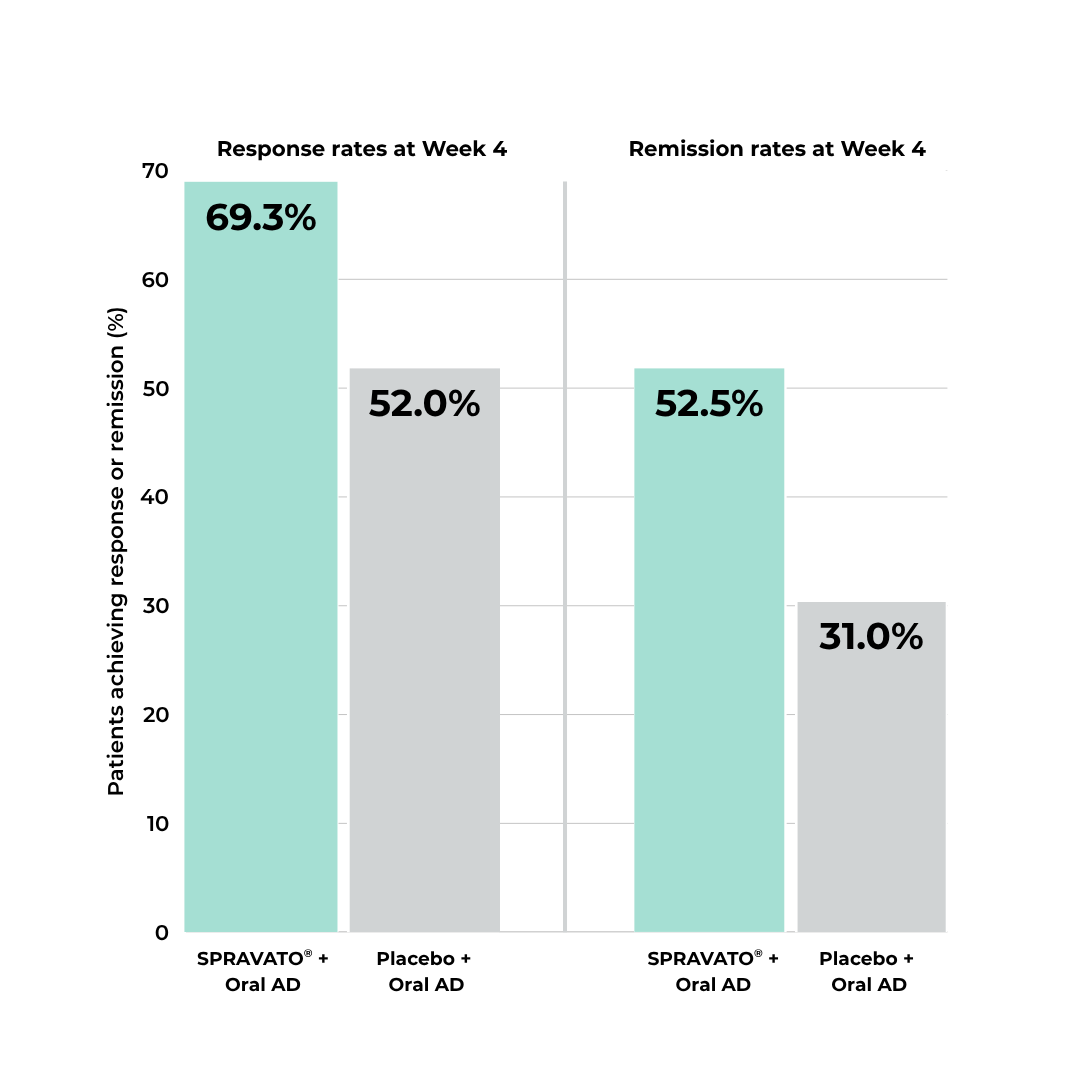
Proven Effective for TRD
In a short-term study, more patients using SPRAVATO®
plus oral antidepressant demonstrated rapid and superior reduction in depressive symptoms at four weeks compared to those who received placebo plus an oral antidepressant.17
Who is SPRAVATO® For?
SPRAVATO® is indicated for the treatment of:
- Treatment-resistant depression (TRD)** in adults as monotherapy or in conjunction with an oral antidepressant.
- Depressive symptoms in adults with major depressive disorder (MDD) with acute suicidal ideation or behavior in conjunction with an oral antidepressant.
**Treatment Resistant Depression defined as inadequate response to two or more oral antidepressants
Identifying a potential SPRAVATO® Candidate
- Patients unsatisfied with results of oral antidepressants
- Patients who have responded to medication, but not in remission
- Patients who have failed at least two different antidepressants from different classes
- Patients who have been diagnosed with MDD or TRD
Not FDA-approved for Bipolar Depression, Schizoaffective Disorder, or Schizophrenia
3 Steps to getting started
Greenbrook Patient Journey

1. No Cost Consultation
Learn about our treatments
After you refer your patient, our team will schedule them a no-cost consultation with one of our Consult Coordinators. The Consult Coordinator will review their history and explain aspects of our treatments.
We take care of the insurance
The Consult Coordinator will review treatment payment options with your patient and walk them through typical insurance requirements. We’ll also coordinate directly with their insurance provider to verify coverage and determine benefits.
2. Pre-Assessment
Find out if treatment is right for your patient
Next, your patient will meet with a Greenbrook-affiliated licensed provider for an evaluation which includes questions about your patient's history and symptoms.
If the provider has determined that your patient is a candidate for treatment and they are ready to move forward, our team will create an individualized treatment plan.

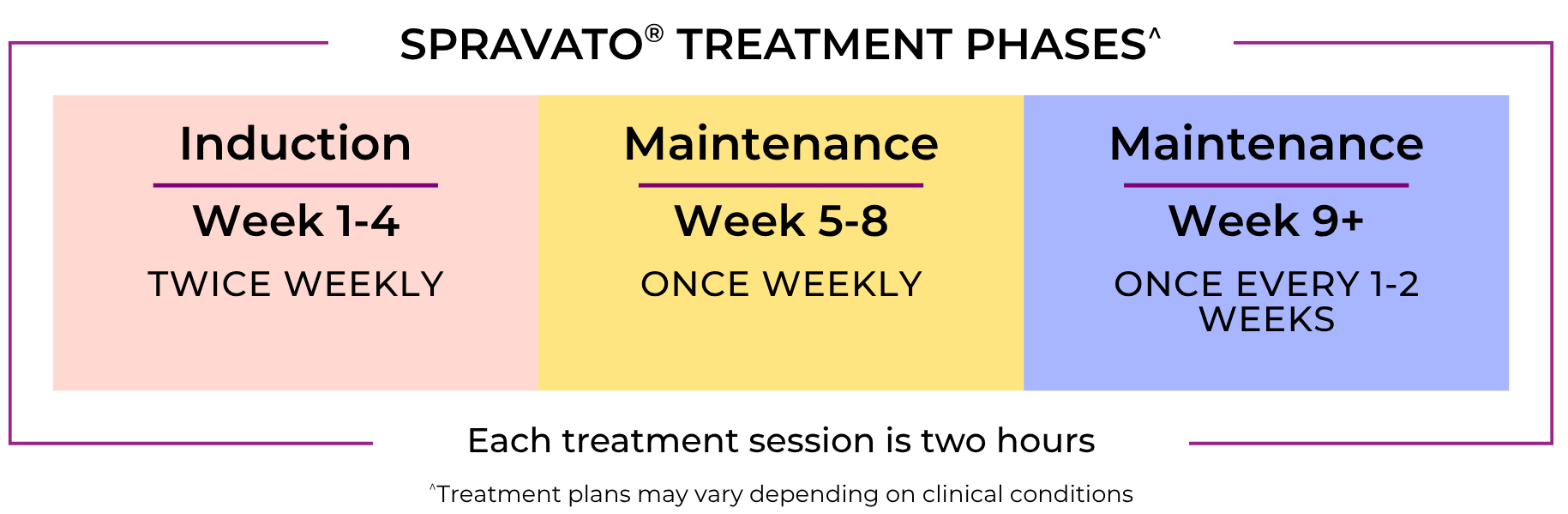
3. Treatment
Depending on your patient's treatment modality they will follow these typical treatment plans
The SPRAVATO® Treatment Experience
The medical staff at Greenbrook’s REMS-certified outpatient treatment centers are trained to prescribe and observe patients treated with SPRAVATO®.

Treatment is provided under the direct supervision and monitoring of trained medical professionals for at least two hours.
Patients should coordinate a ride home after treatment, as they should not drive or operate heavy machinery until the next day, following a restful night’s sleep.
A session lasts as little as 19 minutes per day^^
Resume normal activities immediately after treatment

NeuroStar TMS Therapy Experience
NeuroStar is an easy, in-office experience. Patients are awake and fully alert during treatment.
Do you have a patient who would benefit from treatment with us?
Help them get started today.